Concentrated nitric acid oxidises phosphorus to phosphoric acid according to the following equation: P +5HNO3 → H3PO4 +5NO2+ H2O If 6.2 g of phosphorus was used in the reaction, calculate: a The

Balance the following equation by partial equation method: P4 + HNO3 = H3PO4 + NO2 + H2O | Homework.Study.com

Concentrated nitric acid oxidised phosphorus to phosphoric acid according to the following equation: P+5HNO3(conc.)---> H3PO4 +H2O +5NO2 If 9.3 g of phosphorus was used in the reaction, calculate: I) Number of moles

Oxidation Number method. P4+HNO3+H2O=H3PO4+NO. Balance the equation by oxidation Number method. - YouTube

Oxidation Number method. P4+HNO3+H2O=H3PO4+NO. Balance the equation by oxidation Number method. - YouTube



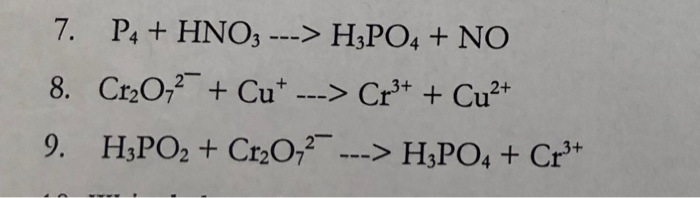



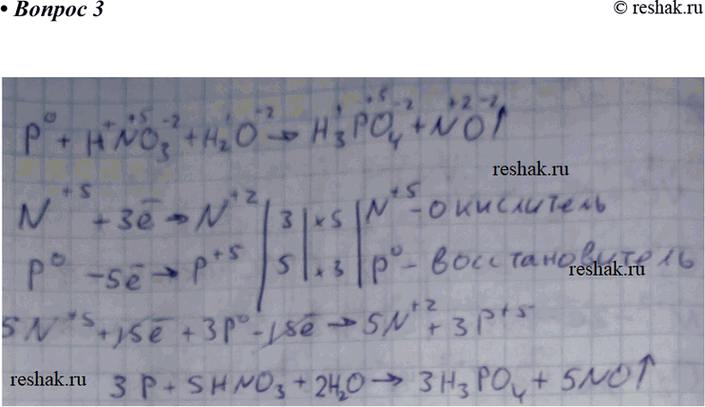




![Método REDOX: HNO3 + P + H2O -> H2PO4 + NO [Solución] Método REDOX: HNO3 + P + H2O -> H2PO4 + NO [Solución]](https://1.bp.blogspot.com/-ZwWwwkGH92Y/XxjdT0PUXpI/AAAAAAAANAw/W9hFhUBgXVc2k6aHKJotGW-xaVjz--cyQCNcBGAsYHQ/s1280/IMG_20200722_151638.png)





